History of HDG
The recorded history of galvanizing goes back to 1742 when a French chemist named P.J. Malouin, in a presentation to the French Royal Academy, described a method of coating iron by dipping it in molten zinc. In 1836, Stanilaus Tranquille Modeste Sorel, another French chemist, obtained a patent for a means of coating iron with zinc, after first cleaning it with 9% sulfuric acid and fluxing it with ammonium chloride. A British patent for a similar process was granted in 1837. By 1850, the British galvanizing industry was using
10,000 tons of zinc a year for the protection of steel. Galvanizing is found in almost every major application and industry where iron or mild steel is used. The utilities, chemical process, pulp and paper, automotive, and transportation industries, to name just a few, historically have made extensive use of galvanizing for corrosion control. They continue to do so today. For over 150 years, hot-dip galvanizing has had a proven history of commercial success as a method of corrosion protection in myriad applications worldwide.
The recorded history of galvanizing goes back to 1742 when a French chemist named P.J. Malouin, in a presentation to the French Royal Academy, described a method of coating iron by dipping it in molten zinc. In 1836, Stanilaus Tranquille Modeste Sorel, another French chemist, obtained a patent for a means of coating iron with zinc, after first cleaning it with 9% sulfuric acid and fluxing it with ammonium chloride. A British patent for a similar process was granted in 1837. By 1850, the British galvanizing industry was using
10,000 tons of zinc a year for the protection of steel. Galvanizing is found in almost every major application and industry where iron or mild steel is used. The utilities, chemical process, pulp and paper, automotive, and transportation industries, to name just a few, historically have made extensive use of galvanizing for corrosion control. They continue to do so today. For over 150 years, hot-dip galvanizing has had a proven history of commercial success as a method of corrosion protection in myriad applications worldwide.
What is corrosion?
Corrosion is the reaction between a material and its environment that produces a deterioration of the material and alters its mechanical properties. The actual corrosion process that takes place on a piece of bare mild steel is very complex due to factors such as variations in the composition/structure of the steel, presence of impurities due to the higher instance of recycled steel, uneven internal stress, or exposure to a non-uniform environment.
 It is very easy for microscopic areas of the exposed metal to become relatively anodic or cathodic. A large number of such areas can develop in a small section of the exposed metal. Further, it is highly possible that several different types of galvanic corrosion cells are present in the same small area of the actively corroding piece of steel. As the corrosion process progresses, the electrolyte may change due to materials dissolving
It is very easy for microscopic areas of the exposed metal to become relatively anodic or cathodic. A large number of such areas can develop in a small section of the exposed metal. Further, it is highly possible that several different types of galvanic corrosion cells are present in the same small area of the actively corroding piece of steel. As the corrosion process progresses, the electrolyte may change due to materials dissolvingin or precipitating from the solution. Additionally, corrosion products might tend to build up on certain areas of the metal. These corrosion products do not occupy the same position in the given galvanic series as the metallic component of their constituent element. As time goes by, there may be a change in the location of relatively cathodic or anodic areas and previously uncorroded areas of the metal are attacked and corrode. This eventually will result in uniform corrosion of the area. The rate at which metals corrode is controlled by factors such as electrical potential and resistance between anodic and cathodic areas, pH of the electrolyte, temperature and humidity.
How do you protect iron and steel from corrosion?
Barrier protection is perhaps the oldest and most widely used method of corrosion protection. It acts by isolating the metal from the electrolytes in the environment. Two important properties of barrier protection are adhesion to the base metal and abrasion resistance.
Cathodic protection is an equally important method for preventing corrosion. Cathodic protection requires changing an element of the corrosion circuit, introducing a new corrosion element, and ensuring that the base metal becomes the cathodic element of the circuit. Hotdip galvanizing provides excellent barrier and cathodic protection. The sacrificial anode method, in which a metal or alloy that is anodic to the metal to be protected is placed in the circuit and becomes the anode. The protected metal becomes the cathode and does not corrode. The anode corrodes, thereby providing the desired sacrificial protection. In nearly all electrolytes encountered in everyday use, zinc is anodic to iron and steel. Thus, the galvanized coating provides cathodic corrosion protection as well as barrier protection.
Service-Life Chart for Hot-Dip Galvanized Coatings
Continous and Batch Galvanize
There are many types of coatings that are specified as hot dip galvanized. The process involves immersing steel in molten zinc. The zinc reacts with the steel to form the galvanized coatings. The time the steel is immersed in the zinc along with post-galvanizing treatment controls the coating thickness, appearance and other characteristics
Hot dip galvanized coatings are applied to steel to improve the anti-corrosion performance of the steel to ensure that it lasts as long as possible with a minimum of maintenance. Standards currently being developed for the housing industry have set a benchmark of at least 50 years as the acceptable life of structural building products. Only hot dip galvanized steel products with the heaviest galvanized coatings are capable of meeting this requirement.
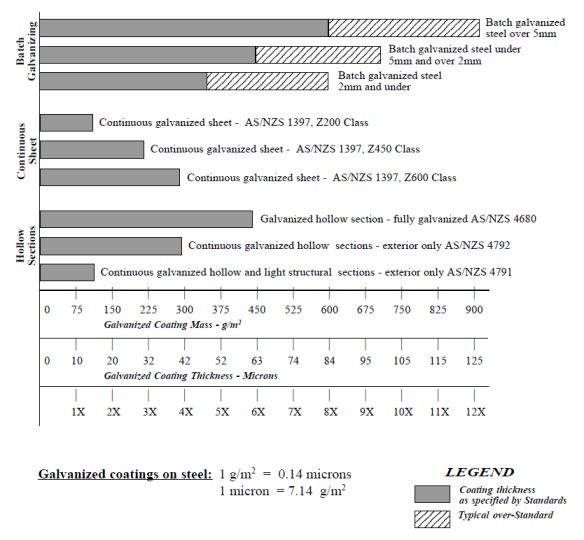
The Australian Standard AS 4680 - 1999 , Hot Dipped Galvanized Coatings on Ferrous Articles, includes galvanized coating standards on sheet, wire, tube and general articles. A great deal of confusion exists through the inclusion of galvanized coatings with significantly different coating characteristics within the same Australian Standard.
COATING THICKNESS COUNTS
All sheet, wire and many tube products are CONTINUOUSLY galvanized. This means that the coating is applied at high speed and the coating thickness is controlled by the process. Immersion time in the zinc is measured in seconds. Alternatively, in the BATCH hot dip galvanizing process steel items are immersed for periods ranging from 3-10 minutes, depending on the mass of the items being galvanized.
These completely different methods of applying galvanized coatings produce different types of coatings.
There are 4 main differences that impact on anti-corrosion performance of BATCH galvanized steel compared to CONTINUOUSLY galvanized steel. These are:
1. Coating thickness - BATCH galvanized items of the same section thickness are typically at least 3 TIMES thicker than similar CONTINUOUSLY galvanized coatings on sheet and tube.
2. Coating hardness - BATCH galvanized items have much thicker zinc/iron alloy layers in the coatings which gives BATCH galvanized items 5 TIMES the abrasion resistance of CONTINUOUSLY galvanized coatings.
3. Coating integrity - BATCH galvanized coatings apply a uniform heavy coating to all internal and external surfaces, edges and cavities. CONTINUOUSLY galvanized coating will always have exposed bare steel at cut edges. CONTINUOUSLY galvanized hollow sections are fully galvanized on the external surfaces only.
4. Coating mass - The cathodic protection of exposed steel by zinc depends of the mass of the zinc in relation to the area of exposed steel. Because of the drainage characteristics of BATCH galvanized coatings, the coating mass on BATCH galvanized products is significantly higher (typically 3-5 times) in proportion to thickness than CONTINUOUSLY galvanized coatings. Hot rolled medium structural sections commonly achieve coating mass levels exceeding 1000 g/m2.
MORE COATING THICKNESS = LONGER COATING LIFE
150 years of field testing has determined that all things being equal, galvanized coating life is equivalent to galvanized coating thickness. When comparing BATCH galvanized coatings to CONTINUOUSLY galvanized coating, all things are not equal.
THE CUT EDGE FACTOR
All CONTINUOUSLY galvanized sections have exposed steel at cut edges and rely on the adjacent zinc in the coating to provide cathodic protection to the bare steel. This requirement accelerates the rate of corrosion of the galvanized coating at cut edges. The thicker the CONTINUOUSLY galvanized section, the faster the rate of coating corrosion at cut edges because of the greater area of bare steel exposed. Even if it was possible to apply a CONTINUOUSLY galvanized coating to a steel item to the same thickness as a BATCH galvanized item, the cut edge factor gives the BATCH galvanized coating a life typically 1.5 TIMES greater.
COMPARISON OF GALVANIZED COATINGS
CONTINUOUSLY galvanized coatings comply very closely to their specified coating mass. BATCH
galvanized coatings on hot rolled steel sections almost always exceed their minimum specified coating
mass.






 :.
:.

 :.
:.










0 komentar:
Posting Komentar